Study Design:
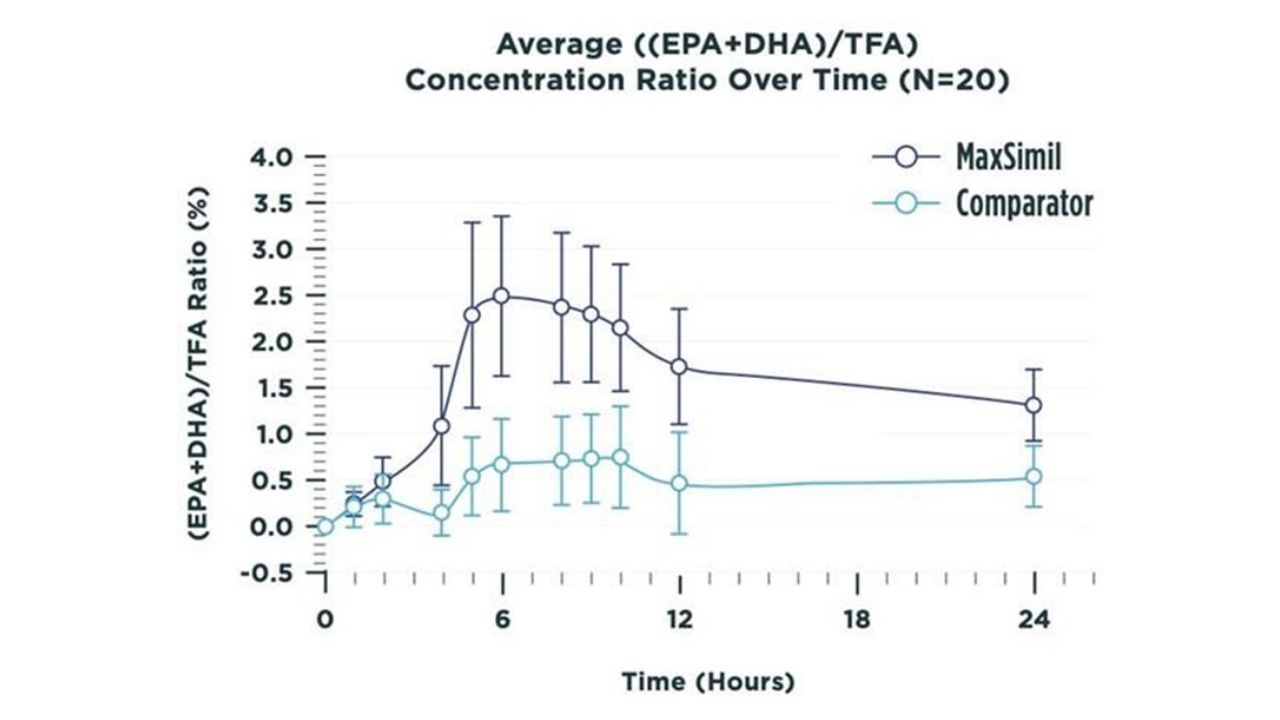
Results:
After receiving the MAG form, plasma EPA and DHA peaked at a concentration 3 and 2.5 times higher,respectively, than with the EE form
Study Design:
Phase 1: Bioavailability Blood samples collected at baseline and multiple intervals (2–72 hours) after a single dose of Vitamin K1 or Menatto
Phase 2: Dose Dependence Blood samples taken 4 and 24 hours after single doses (50-500 mcg) of Menatto with a 2-week washout between doses
Phase 3: Osteocalcin Carboxylation Participants took Vitamin K1 or Menatto for 6 weeks, measuring the ratio of carboxylated (cOC) to uncarboxylated osteocalcin (ucOC)
Phase 4: Interaction with Anticoagulants Various Menatto doses tested in participants treated with an anticoagulant drug
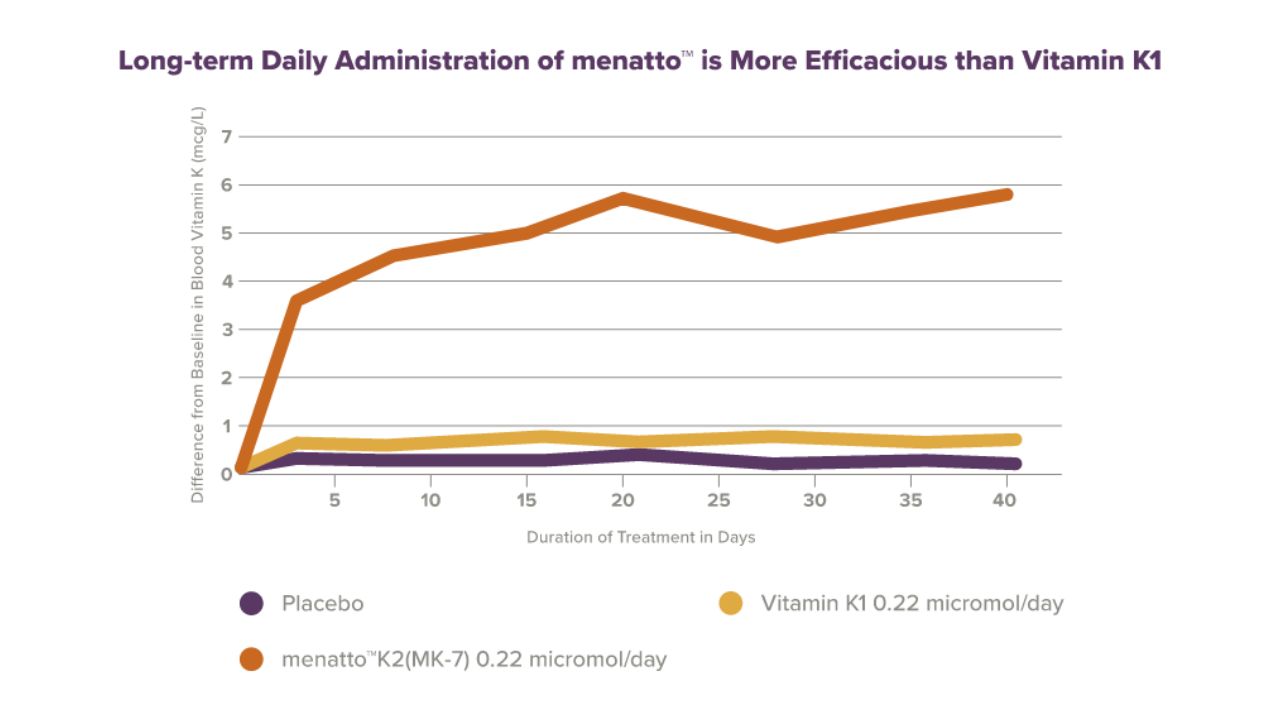
Results:
menatto® had a much longer half-life than vitamin K1, which allowed it to achieve more stable and much higher blood levels (7 to 8-fold higher) after prolonged intake
Study Design:
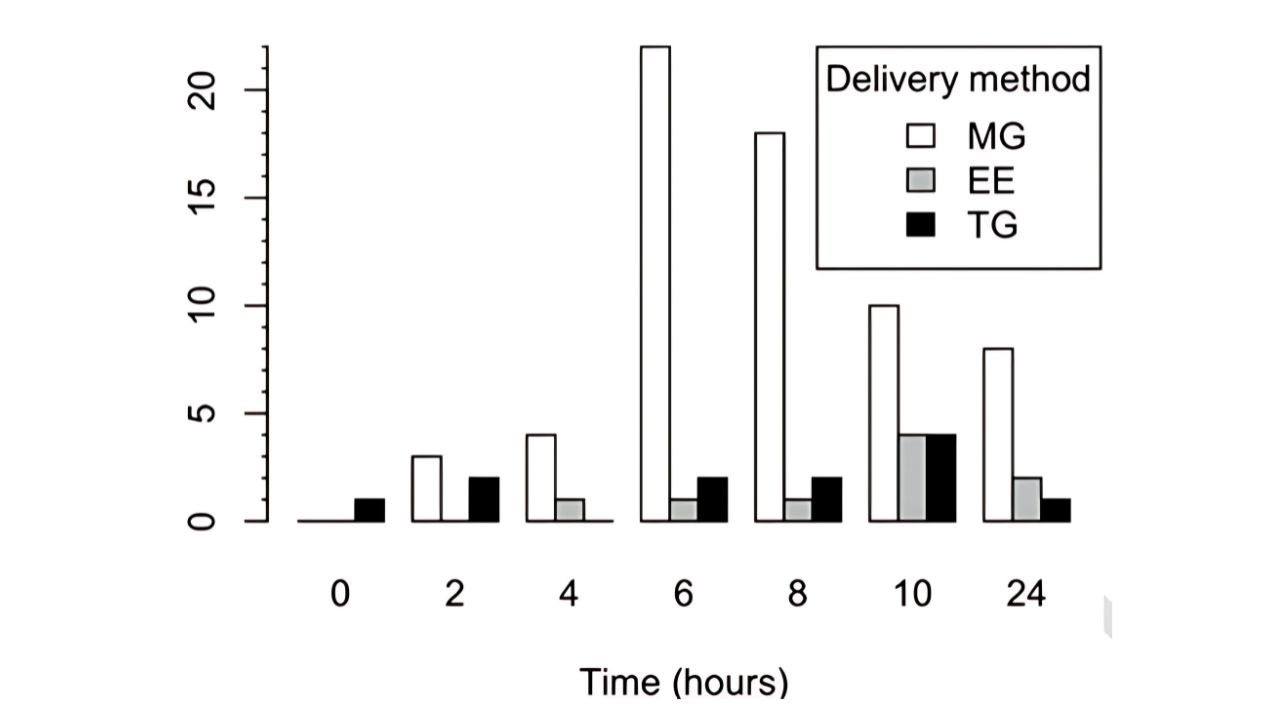
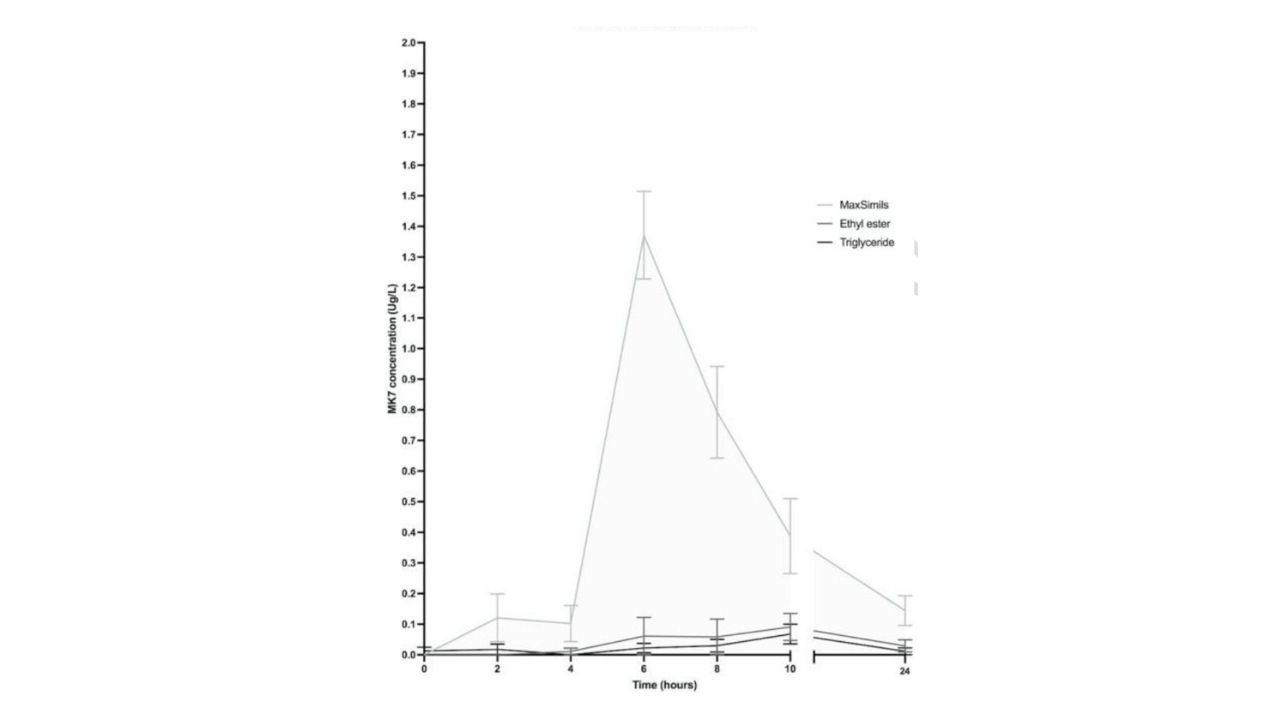
Results:
The 24-h AUC of plasma MK-7 following the MAG supplementation was ~7 and ~11 times higher than those for the EE (P < 0.001) and TG (P<0.001)
conditions.
GCViDeal is an Innovation Licensing firm to commercialize global healthcare and life science innovations in India & other developing countries. We expedite innovation commercialization by out licensing it to the top marketing companies on private label.
Copyright © 2023 GCVideal. All rights reserved. A brand owned by GCV Life Pvt. Ltd.